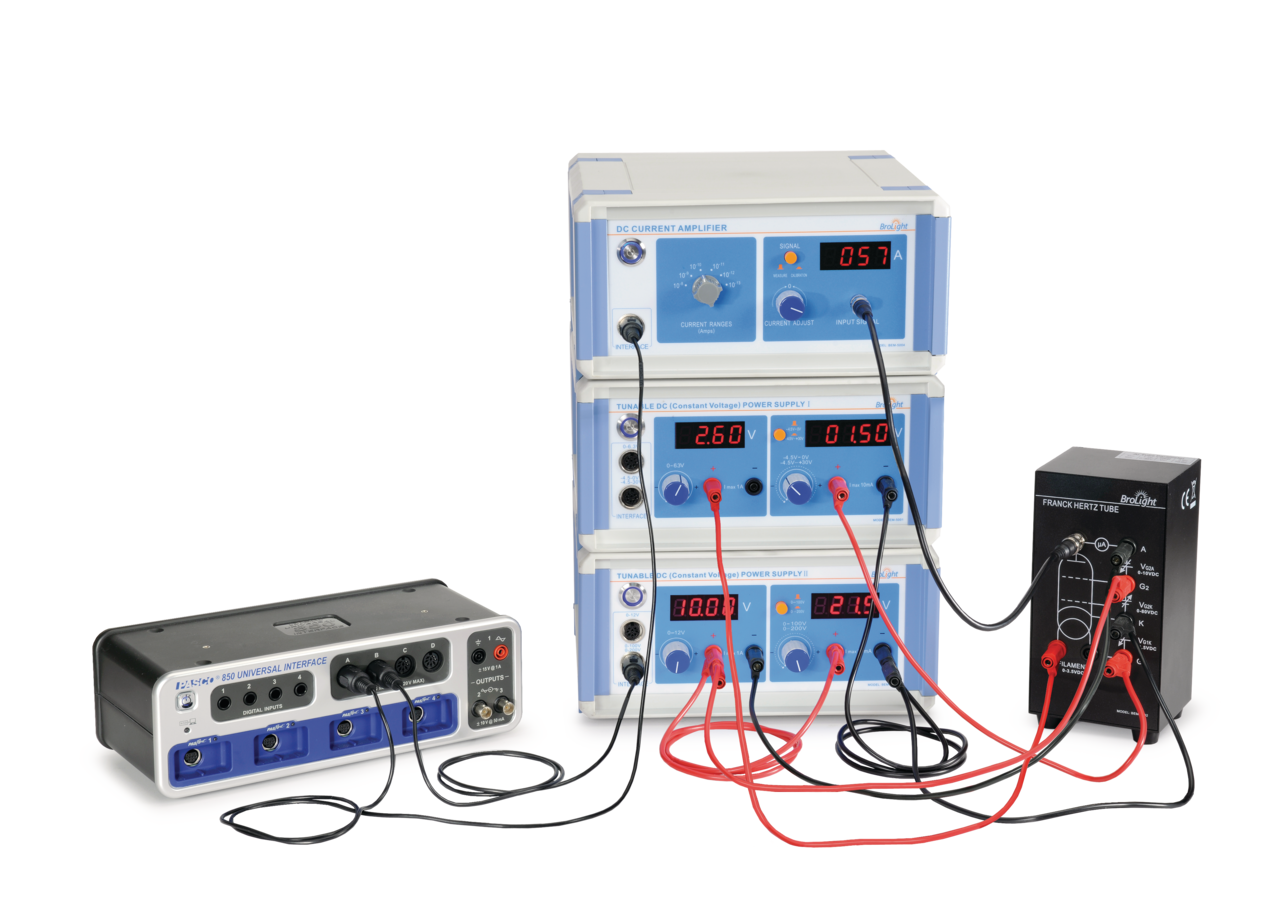
Description
As early as 1914, James Franck and Gustav Hertz discovered in the course of their investigations an energy loss in distinct steps for electrons passing through mercury vapor and a corresponding emission at the ultraviolet line (λ= 254 nm) of mercury. They performed this experiment that has become one of the classic demonstrations of the quantization of atomic energy levels. They were awarded the Nobel Prize for this work in 1925.
How It Works
Electrons are accelerated by applying a known potential between two grids inside the argon tube. When an electron has sufficient kinetic energy to excite one of argon’s outer orbital electrons and has an inelastic collision with an argon atom, the electron loses a specific amount of kinetic energy. This loss of electron kinetic energy causes a decrease in the electron current in the argon tube. Within a very short time, the excited argon electron will fall from the excited state back into the ground state level, emitting energy in the form of photons.
As the accelerating voltage is increased, the electrons undergo multiple collisions and the excitation energy of the argon atom can be determined by the differences between the accelerating voltages that cause a decrease in the current. Planck‘s Constant can be determined.
Lab Activities & Experiments
Franck Hertz Experiment (Capstone)
Frank Hertz with Data (Capstone)
What’s Included
- 1x Franck-Hertz Tube Enclosure with Argon Tube (SE-9650)
- 1x DC Power Supply I (Constant Voltage) (SE-6615)
- 1x DC Power Supply II (Constant Voltage) (SE-9644)
- 1x DC Current Amplifier (SE-6621)
- 1x Red and Black Patch Cords
Product Specifications
| Filling Gas | argon |
| Filament Voltage | ≤6.3 VDC |
| Accelerating Voltage | ≤100 VDC |
| Wave Crest (or Trough) Number | 6 |
| Argon Tube Life Span | ≤3000 hrs |
Support Documents
Franck Hertz Experiment Manual